Human trials for revolutionary teeth regeneration drug set to begin
Trials to begin for a teeth regeneration drug whose commercial availability could be possible by 2030
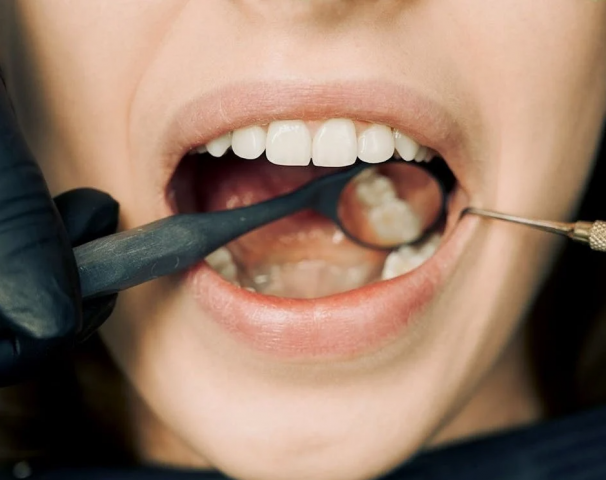
Japanese researchers at Kyoto University Hospital are ready to begin human trials for a revolutionary teeth regeneration drug.
This drug aims to suppress the uterine sensitization-associated gene-1 (USAG-1) protein, an antibody that prevents tooth regrowth.
The initial human trial, scheduled from September 2024 to August 2025, will involve 30 men aged between 30 and 64 who are missing at least one molar.
If successful, the study will expand to include individuals with partial edentulism, or those missing one to five permanent teeth.
The promising results from tests on ferrets and mice have shown no notable side effects, according to Popular Mechanics.
If the trials prove effective, the medicine could be commercially available as soon as 2030.
This breakthrough could eliminate the need for dentures and dental implants, offering a permanent solution for those who have lost teeth due to various reasons.
"We want to do something to help those who are suffering from tooth loss or absence,” said lead researcher Katsu Takahashi, head of dentistry and oral surgery at Kitano Hospital, as reported by NewAtlas. "While there has been no treatment to date providing a permanent cure, we feel that people’s expectations for tooth growth are high."
The intravenous treatment works by suppressing the USAG-1 protein, which has been identified as a key inhibitor of tooth regrowth. In previous animal studies, this approach allowed for the regeneration of teeth without significant adverse effects.
The upcoming human trials aim to replicate these results and pave the way for a viable treatment for tooth loss.



















COMMENTS (2)
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ