Britain approves Merck's oral Covid pill in world first
Merck expecting to produce 10 million courses of treatment by end of this year
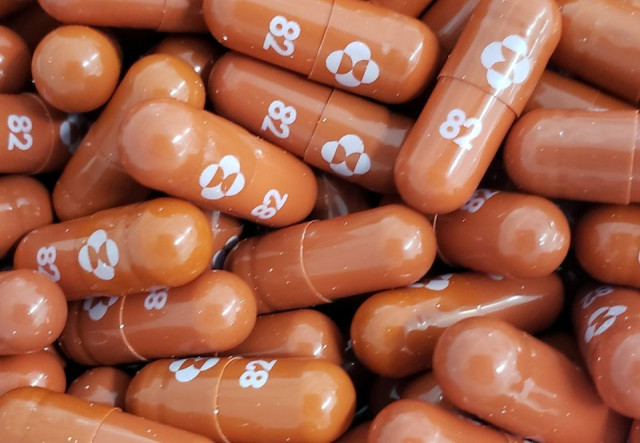
Britain on Thursday became the first country in the world to approve a potentially game-changing Covid antiviral oral pill jointly developed by Merck and Ridgeback Biotherapeutics, in a boost to the fight against the pandemic.
The Medicines and Healthcare products Regulatory Agency (MHRA) recommended that the drug, molnupiravir, be used as soon as possible following a positive Covid test and within five days of the onset of symptoms.
Read Analysis: Country by country, scientists eye beginning of an end to Covid pandemic
The government and the NHS will confirm how this Covid treatment will be deployed to patients in due course.
Separately, Merck said it was expecting to produce 10 million courses of the treatment by the end of this year, with at least 20 million courses set to be manufactured in 2022.



















COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ