DUHS scientists await clinical trials for COVID-19 treatment
Devise intravenous immunoglobulin with plasma obtained from plasma of recovered patients
KARACHI: Researchers at the Dow University of Health Sciences (DUHS) have prepared intravenous immunoglobulin (IVIG) with plasma obtained from the blood of patients recovered from COVID-19, in an important breakthrough in the global fight against coronavirus.Many countries across the world are holding clinical trials for plasma therapy or transfusion to fight the novel virus. However, the treatment devised by the DUHS scientists is safer and more effective because as it does not carry the undesired component of blood, they say.
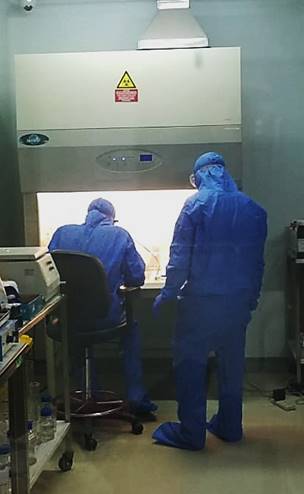 Specialists at the DUHS research facility. PHOTO - EXPRESS
Specialists at the DUHS research facility. PHOTO - EXPRESSThe IVIG will now be sent to the Drug Regulatory Authority (DRAP) to for its permission for the clinical trials and then to the National Bioethics Committee (NBC) for approval, said a statement issued here on Monday.
DRAP has already allowed the clinical trial of passive immunisation technique, using the plasma obtained from the donors, who have recovered from Covid-19. This treatment had been used globally to curb other viral epidemics.
DRAP approves passive immunisation technique for treating COVID-19
The IVIG is devised at the DUHS after several chemical processes. It can be administered through an injection. The clinical trial will determine how best it could be administered, its effects on kidneys and other side-effects. After the successful trials, the IVIG could be available for general use.
 A researcher prepares the IVIG at the DUHS. PHOTO: EXPRESS
A researcher prepares the IVIG at the DUHS. PHOTO: EXPRESSExperts related to the pharmaceutical industry have hailed the country’s ability to devise immunoglobulin but remained sceptical about DRAP’s capacity to check the quality of the antibodies obtained from plasma.
They said that DRAP has no central laboratory to check the immunoglobulin prepared by the DUHS scientists. DRAP does not have any plant to check the efficacy of any bioproduct, they added.

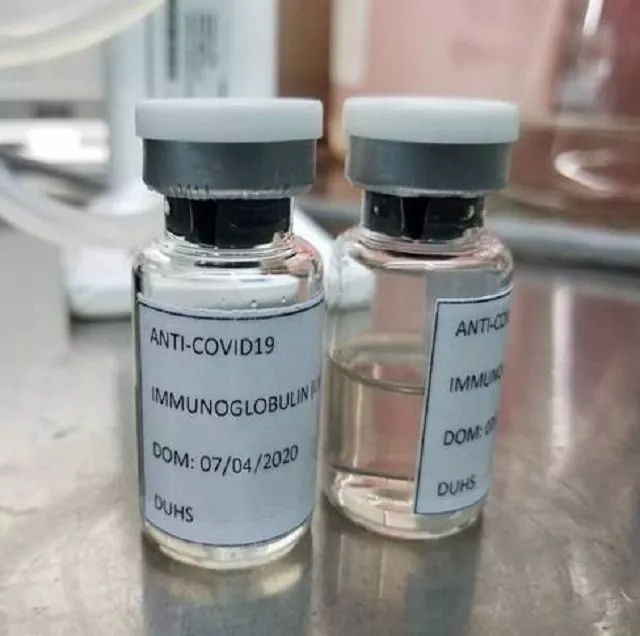
COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ