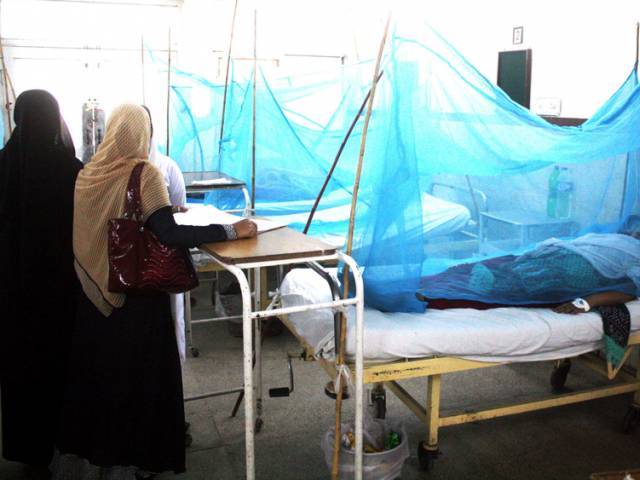
It was September, Khyber-Pakhtunkhwa (K-P) was reeling with one of its worst outbreaks of dengue. Thousands had shown symptoms and had tested positive for the disease while hundreds had been admitted to overflowing hospitals and 12 dead.
With the high court breathing down their neck, the government decided to opt for what promised to be a magic wand which could wish away their problems – a vaccine.
Dengue is spreading rapidly in many parts of the world, especially in third world countries where it is endemic – like Pakistan. While not perceived to be deadly, dengue kills around 20,000 people around the world every year.
The World Health Organisation (WHO), the apex global health body, recommended that countries chronically suffering from outbreaks should resort to vaccination. The Dengvaxia vaccine, produced by Sanofi Pasteur, a division of French pharmaceutical firm Sanofi, is the first-ever vaccine for dengue.
But before officials in Peshawar decided to opt for it, Philippines was among only a handful of countries in the world which had opened its clinics to the vaccine where children as young as nine were vaccinated in a two-year national effort against the disease.
Desperate, health officials from K-P’s health ministry wrote to the apex drug regulator in the country, the Drug Regulatory Authority of Pakistan (DRAP), with a request to grant a special concession to register Dengvaxia so that it could be legally imported in the country. In view of the extreme situation, DRAP obliged, approving the vaccine on an “immediate and emergency basis”.
But even as the toll ballooned to 70 in subsequent months, there was little news about the vaccine being used locally and it took a cold turn to the weather to naturally control the outbreak.
Then, disaster.
In late November 2017, Sanofi globally issued details of a new analysis from six years of clinical data which showed that Dengvaxia behaves differently in those who had been previously infected with Dengue and those who had not.
“The analysis confirmed that Dengvaxia provides persistent protective benefit against dengue fever in those who had a prior infection,” Sanofi said in a globally issued statement on November 29.
“For those not previously infected by dengue virus, however, the analysis found that in the longer term, more cases of severe disease could occur following vaccination upon a subsequent dengue infection,” it warned, urging healthcare professionals to assess the likelihood of prior dengue infection in an individual before vaccinating, recommending its use only when the potential benefits outweigh the potential risks.
“For individuals who have not been previously infected by dengue virus, vaccination should not be recommended,” it said.
Days later, health authorities in the Philippines halted their vaccination programme, a move supported by the WHO. The president’s office, on the back of growing calls from lawmakers and reports of deaths among the vaccinated, launched an investigation into the vaccine’s impact.
It sent the K-P health ministry into a frenzy.
“We came to know that the vaccine had been imported by some other countries where it caused deaths, probably more than those who became a victim of the virus,” a senior health official dealing with the issue told The Express Tribune on the condition of anonymity.
All arrangements for importing and disseminating the vaccine were nearly finalized when officials withdrew their decision, but not before they found out that the vaccine would not only cost too much but could lead to more deaths, not to mention that the vaccine was only effective against two of the four types of the dengue virus prevailing in the province.
A senior official questioned the motives for the vaccine, especially “if you know the repercussions, why would you request for its import?”
The health department, though, defended its decision.
“Not at all, we just discussed it as an option,” explained Dr Shaheen, the head of the public health section at the health department.
“[We] never wanted to import it after we reviewed things [clinical study],” the official told The Express Tribune.
Noting that they had consulted the company as well as experts from the WHO, she confirmed some countries had imported the vaccine leading to complications.
“No, we do not have any plan to import the vaccine,” Dr Shaheen clarified.
On the other hand, Health Services Director-General Dr Ayub Roz shared that one patient required three shots of the dose but still did not display the required results.
“Since we do not have any facility to certify whether it [vaccine] was safe or not, we don’t think it could be imported,” Dr Roz told The Express Tribune adding, “the vaccine cannot be used for every case.”
When asked about the letter written to DRAP, Roz stated the letter was written to import and certify the vaccine, but since there are no facilities to certify the vaccine, there was little chance the vaccine could be imported.
Meanwhile, a local representative of Sanofi confirmed to The Express Tribune that the company had issued a “label update” advisory for the vaccine.
“Sanofi is asking regulatory authorities to update information provided to physicians and patients on its dengue vaccine. This label update is for countries where the vaccine is approved,” wrote Laila Khan, the director for External Affairs at Sanofi Pakistan, in an emailed response.
“Sanofi’s vaccine for dengue is not yet approved/registered by DRAP for availability in Pakistan in the private market. However, Sanofi Pakistan has proactively informed DRAP as well.”
Based on these recommendations and reviewing WHO reports and guidelines, the registration board of DRAP surprisingly approved the drug for import in its last meeting for 2017. The board, though, noted that the vaccine was not meant for routine immunization and would only be used for passive vaccination of patients who have suffered and recovered from dengue.
Published in The Express Tribune, January 4th, 2018.



















1714119118-0/image-(7)1714119118-0-270x192.webp)






1714024018-0/ModiLara-(1)1714024018-0-270x192.webp)









COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ