Scientists convert spinach leaves into human heart tissue — that beats
The leaf vascular structure was seeded with heart muscle cells
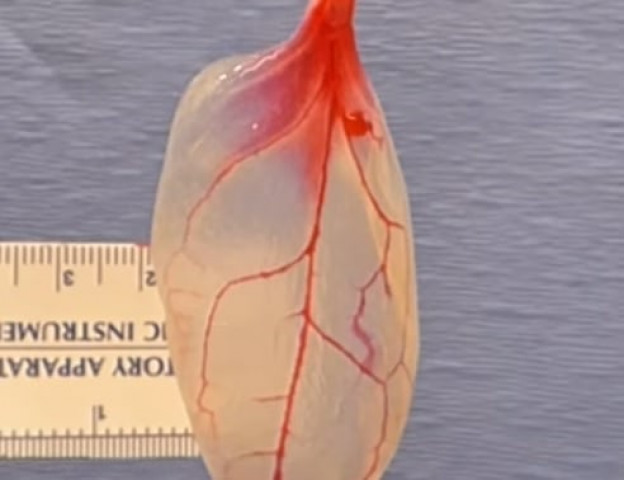
The leaf vascular structure was seeded with heart muscle cells and after a few days of wait, the heart cells suddenly contracted mirroring the movement of a human tissue. PHOTO: SCREENSHOT
Researchers from Worcestor Polytechnic Institute [WPI] used the leaf’s own network of veins to develop a network of cells that supports human functions by first stripping the plant off green material until the only thing left was fine cellulose structure holding the leaf in one place.
The leaf's vascular structure was seeded with heart muscle cells and after a few days of wait, the heart cells suddenly contracted mirroring the movement of a human tissue.
"Plants and animals exploit fundamentally different approaches to transporting fluids, chemicals, and macromolecules, yet there are surprising similarities in their vascular network structures," the scientist wrote in their research paper published in the Biomaterials Journal.
Scientists discover new organ in human body
The process, known as decellularisation, involves using a detergent solution that washes away plant cells. The cellulose, in plants, was found to be compatible with living tissue in previous studies, it is also cheap to buy or grow. The scientists bought spinach for this study at their local market.
Spinach, according to the study, was the closest to a heart tissue since it has a high concentration of vessels.
"I had done decellularisation work on human hearts before, and when I looked at the spinach leaf its stem reminded me of an aorta," says lead researcher Joshua Gershlak. "So I thought, let's perfuse right through the stem. We weren't sure it would work, but it turned out to be pretty easy and replicable. It's working in many other plants."
 A seven day follow up of the spinach leaf. PHOTO: SCIENCE ALERT
A seven day follow up of the spinach leaf. PHOTO: SCIENCE ALERTFor a long time, researchers have been working on growing organs in the lab to combat a shortage of donor organs but until now they relied mostly on 3D printing. This new find can be useful for patients with damaged heart tissue.
"The idea here is that we have this very thin, flat piece of tissue that already has a vascular network in there, so we should be able to potentially stack up multiple leaves and create a piece of cardiac tissue," Gershlak added.
The team is currently researching a way to integrate the leaf with a living human heart tissue.
"Currently, it is as yet unclear how the plant vasculature would be integrated into the native human vasculature and whether there would be an immune response," the research paper explains.
Scientists create first artificial mouse 'embryo' from stem cells
"We really believe that this scaffold has the capability to help treat patients," said the biomedical researcher who runs the WPI lab, Glenn Gaudette. "We have a lot more work to do, but so far this is very promising."
"To be able to just take something as simple as a spinach leaf, which is an abundant plant, and actually turn that into a tissue that has the potential for blood to flow through it, is really very very exciting, and we hope it's going to be a significant advancement in the field," he added.
The scientists used the same technique on parsley, sweet wormwood and roots of a peanut plant during the experiment, hoping that in the future different plants can be used to replicate different tissues. They found that the structure of wood could be “useful in bone engineering”.
Watch the video here:
This article originally appeared on the Science Alert.



















COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ