
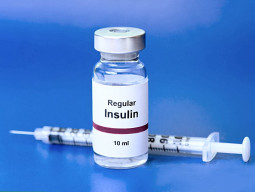
Democratic US Senate aides will meet with Novo Nordisk executives on Tuesday to discuss fallout from its decision to stop selling one of its long-acting insulins in the country, two sources familiar with the meeting told Reuters.
Novo Nordisk will meet with the aides for Senators Jeanne Shaheen, Raphael Warnock, and Elizabeth Warren. In April, the lawmakers wrote to the company expressing alarm at its decision, announced in November, that it would permanently discontinue Levemir by the end of 2024.
Novo said it has given patients enough time to switch to other options, according to a May letter seen by Reuters. The company is unaware of plans for drug manufacturers to produce a biosimilar version of the insulin, the letter said, adding Novo would not assert any patent against such a version.
The sources declined to be named citing the sensitivity of the matter.
It was not clear who from Novo would attend the meeting. A company spokesperson denied there was a meeting scheduled. Shaheen's office confirmed a meeting with Novo was scheduled but declined to provide details. Warnock's and Warren's offices did not respond to a request for comment. Meetings between Congressional aides and company representatives are routine.
The Novo spokesperson said in a statement to Reuters on Friday that Levemir was not discontinued due to "success" of the company's newer medicines Wegovy and Ozempic, widely prescribed for weight loss and part of a new class of drugs called GLP-1 agonists.
When announcing in November the discontinuation of Levemir in the U.S., Novo Nordisk cited manufacturing constraints, reduced patient access and available alternatives -- including its other long-acting insulin Tresiba.
This prototype prosthetic foot is designed to simulate the anatomy of a real human foot.
The company said a number of long-acting insulins remained on the market, but experts say it can be inconvenient and stressful for people with diabetes to change insulin regimens
Novo has acknowledged during investor calls at quarterly earnings in the past year that it faces manufacturing capacity limitations for some products as it races to increase production of Wegovy and Ozempic.
The company along with rivals Eli Lilly and Sanofi make 100% of all insulin sold in the US.
Last year all three companies agreed to cut US list prices for insulin products by up to 75% in 2024, responding to political pressure to make the life-sustaining diabetes treatments more affordable.
Scrutiny of Novo has increased at a time of record profits thanks to commercial success of Wegovy, launched in the US in 2021. Sales of that weight-loss injection propelled the company to the position of Europe's most valuable company worth 587 billion euros ($640 billion).
CEO Lars Jorgensen will testify before the Senate Committee on Health, Education, Labor and Pensions (HELP) in September on the high US costs of Wegovy and Novo's diabetes drug Ozempic.
On Wednesday, Senator Bernie Sanders told Reuters he was confident that Novo can be convinced to cut prices after he publicly shamed the company over how its U.S. prices far surpass those charged in other countries.
Levemir's U.S. sales were 1.27 billion Danish crowns ($185.6 million) in 2023. Sales of Novo's other long-acting insulin Tresiba were 1.33 billion crowns last year.



















COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ