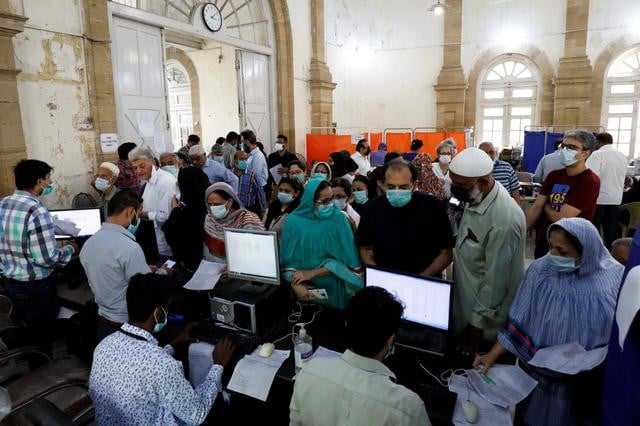
Vaccination drive for citizens aged 19, above to commence from tomorrow
NCOC chairman says registration marks opening of inoculation for 'entire population'
ISLAMABAD:
National Command and Operation Centre (NCOC) chief Asad Umar announced on Wednesday opening Covid-19 vaccine registration for citizens aged 19 years and above from tomorrow (Thursday).
The minister termed the start of the vaccination drive for the age group as marking the opening of inoculation for the entire population.
"In today's NCOC meeting, we decided to open up vaccination registration for all 19 years and above," Umar said in a tweet.
"This registration will start from tomorrow. So now, registration will be open for the entire national population which is approved by health experts for Covid vaccination," he added.
In today's NCOC meeting we decided to open up vaccination registration for all 19 years and above. This registration will start from tomorrow. So now registration will be open for the entire national population which is approved by health experts for covid vaccination
— Asad Umar (@Asad_Umar) May 26, 2021
Two days ago, on May 24, in a major achievement in its fight against the novel coronavirus, Pakistan announced that it has developed a homemade vaccine — PakVac — with the help of China's Cansino Bio after rigorous quality control checks.
The step is aimed at significantly reducing Pakistan’s dependence on other countries for the Covid-19 vaccine.
The country started its vaccination campaign in February with doses donated by the government of China. The campaign started with frontline healthcare workers and then inoculation of senior citizens in the second phase.
Read Single dose CanSino vaccine to be available for citizens by May end
"Congratulations to the NIH (National Institute of Health) Pak team and its leadership for successful fill/finish (from concentrate) of the Cansino vaccine with the help of Cansino Bio Inc. China," Special Assistant to Prime Minister on Health Dr Faisal Sultan wrote on his official Twitter handle.
He said that the vaccine has passed rigorous internal quality assurance testing, considering it an important step to help the vaccine supply line.
An official from the Ministry of National Health Services has said that due to the agreement on technology transfer, the NIH would be able to produce three million doses per month.
1726722687-0/Express-Tribune-Web-(9)1726722687-0-270x192.webp)














COMMENTS (1)
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ