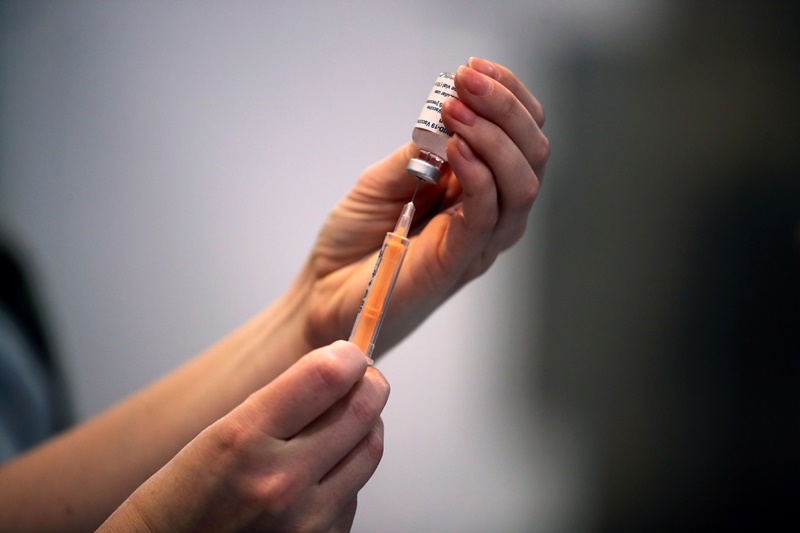
The University of Oxford has launched a study to assess the safety and immune response of the Covid-19 vaccine it has developed with AstraZeneca Plc in children for the first time, it said on Saturday.
The new mid-stage trial will determine whether the vaccine is effective for people between the ages of 6 and 17, according to an emailed statement from the university.
Around 300 volunteers will be enrolled and first inoculations are expected this month, Oxford said.
The two-dose Oxford/AstraZeneca vaccine has been hailed as a ‘vaccine for the world’ because it is cheaper and easier to distribute than some rivals.
AstraZeneca has a target to produce 3 billion doses this year and aims to produce over 200 million doses per month by April.
Also Read India, pharmacy of the world, falls behind on vaccinations at home
1731570357-0/elon-musk-(1)1731570357-0-405x300.webp)

1731673687-0/BeFunky-collage-(63)1731673687-0-165x106.webp)





1731658159-0/BeFunk_§_]_-(19)1731658159-0.jpg)
1731653601-0/Copy-of-Untitled-(43)1731653601-0-270x192.webp)







COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ