Chinese pharmaceutical company's Covid-19 vaccine will be ready by end of the year
Covid-19 vaccine will cost less than $140
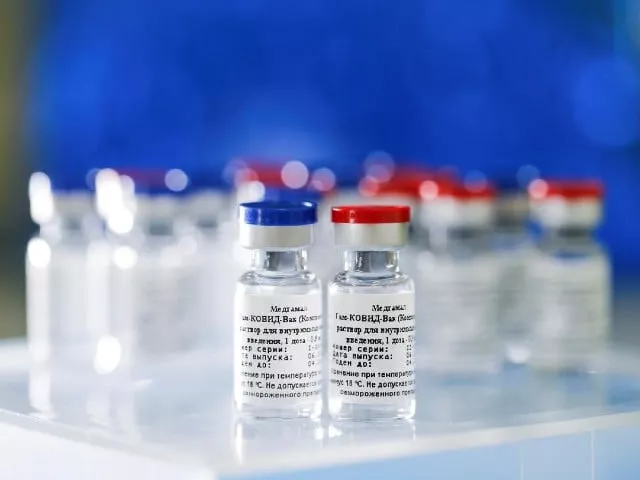
It has been reported that the new type of coronavirus vaccine produced by a state-owned pharmaceutical company in China will be ready to be released by the end of the year.
Liu Cingcin, the director of SinoPharm company, told the Chinese Communist Party's Guangming newspaper that the Covid-19 vaccine, which will be released at the end of the year, will cost less than $ 140 and that two doses will be made 28 days apart.
Google Maps is getting a lot more detail, colourful imagery
Liu stated that workers and students living in large cities would need to be vaccinated, but there was no such requirement for those in rural areas with any densely populated population. "Not all of our 1.4 billion people have to get vaccinated," said Liu Cingin. he spoke.
Stating that he also administered this vaccine, SinoPharm's manager also noted that the company has the capacity to produce 220 million doses per year.
The news that Chinese experts and managers were shot by vaccines in the trial phase has been the subject of discussion in scientific circles.
Beyond batteries: Scientists build methanol-powered beetle bot
The number of people detected with a new type of coronavirus (Covid-19), which emerged in Wuhan, China's Hubey province and spread to the world, has exceeded 22 million 52 thousand worldwide.
According to the "Worldometer" website, where updated data on new cases in countries and regions with Covid-19 were compiled, 777 thousand 477 people died worldwide due to the virus.

COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ