NIBD's passive immunisation technique gets US drug agency's nod for clinical trials
It's mandatory to get approval from international health institutions before human trials, says Dr Tahir Shamsi
KARACHI: In a major development, the United State's Food and Drug Administration (FDA) on Saturday registered the passive immunisation technique from the National Institute of Blood Diseases (NIBD) to treat Covid-19 patients.The technique is used when there is a high risk of infection and insufficient time for the body to develop its own immune response, or to reduce the symptoms of ongoing or immunosuppressive diseases.
Convalescent plasma taken from a recovered patient is believed to be rich in the antibodies needed to fight off the deadly infection.
Researchers around the world register their protocols at ClinicalTrials.gov, being run by America's FDA and NIH institutions.
Experts from across the globe discuss and coordinate with each other regarding their work on the forum.
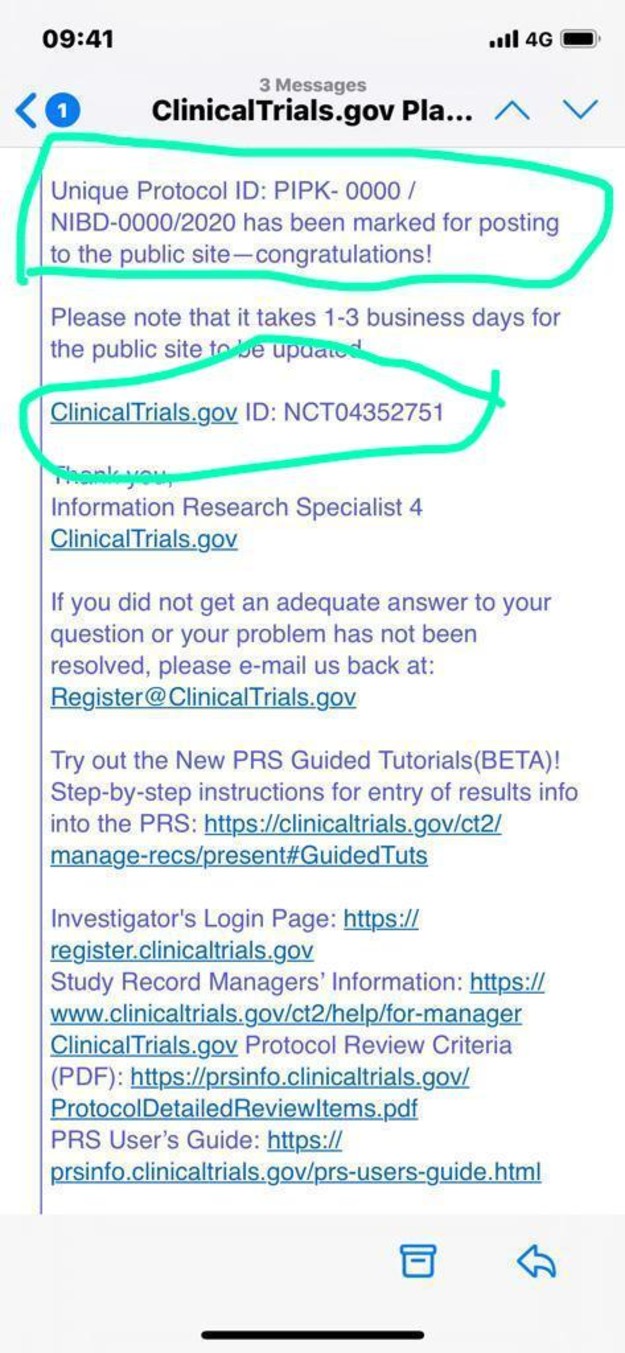 SCREENSHOT OF EMAIL RECEIVED FROM THE FDA
SCREENSHOT OF EMAIL RECEIVED FROM THE FDAProfessor Dr Tahir Shamsi, renowned haematologist and the head of NIBD, told The Express Tribune that FDA has registered the clinical protocol of the technique, adding that the World Health Organisation (WHO) has also been informed.
He said that it was mandatory to get approval from international health institutions before the technique could be administered on humans.
'No chance of contracting virus again'
Dr Shamsi said the research on plasma samples, acquired from the recovering patients in Karachi, has also been completed. "Amazingly, we found [during the research] that there was no chance that the donors could contract the coronavirus again in future which was a very encouraging sign," he added.
Meanwhile, a meeting regarding the clinical trials of passive immunisation technique was chaired by Sindh Health Minister Azra Fazal Pechuho. Chief Technical Advisor Dr Aijaz Khan, Director Sindh Blood Transfusion Authority Dr Durre Naz Jamal, Professor Khalid Shamsi, Dr Tahir Shamsi and others attended the meeting.
Recovery from coronavirus may not confer immunity, warn experts
The Sindh Health Ministry granted permission to administer the technique on a trial basis at the government-approved health institutions across the province.
The participants of the meeting were informed that authorities at NIBD in Karachi, Liaquat University in Jam Shoro and Regional Blood Centre in Sukkur would be responsible for collecting blood plasma from the recovering patients.
Dr Durre Naz Jamal directed NIBD to give training and establish the centres at Liaquat University in Jam Shoro and Regional Blood Centre in Sukkur for blood plasma collection.
The Drug Regulatory Authority of Pakistan (DRAP) has already approved the passive immunisation technique.
Dr Shamsi, in an earlier interview with The Express Tribune, had said that the recovered patients can donate blood plasma after every two weeks which could save the life of at least one patient.
NIBD set to treat COVID-19 patients through passive immunisation
“NIBD in Karachi is equipped with four machines which can extract plasma of 40 patients per day,” Dr Shamsi said.
According to him, health condition of coronavirus patient will start improving within 48 hours after undergoing treatment and in the next six days, the patient will be recuperated from the mysterious illness.
The country’s top haematologist and transplant surgeon also said that Rs50,000 expenses are incurred for extracting plasma from one individual but NIBD will do it free of charge.

COMMENTS
Comments are moderated and generally will be posted if they are on-topic and not abusive.
For more information, please see our Comments FAQ